Win Ratio References
By Tuo Wang in Clinical Trial Survival Analysis
January 15, 2021
Win Ratio References
Win ratio is a novel approach to analyze prioritized composite endpoints. After proposed by Pocock et al. (2012), it gained much attention in both academic and industry. I summarized some important references regarding win ratio statistics.
Last updated date: April 2023
Theory
Background:
-
Combining mortality and longitudinal measures in clinical trials., Finkelstein and Schoenfeld (1999)
-
Generalized pairwise comparisons of prioritized outcomes in the two-sample problem., Buyse (2010)
Original paper: The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities, Pocock et al. (2012)
Inference for win ratio:
-
The asymptotic distribution of the Net Benefit estimator in presence of right-censoring, Ozenne (2021).
-
Large sample inference for a win ratio analysis of a composite outcome based on prioritized components, Bebu and Lachin (2015)
-
An alternative approach to confidence interval estimation for the win ratio statistic, Luo et al. (2015)
-
Evaluation of inferential methods for the net benefit and win ratio statistics, Verbeeck (2019)
-
Inference on win ratio for cluster-randomized semi-competing risk data, Zhang and Jeong (2021)
Alternative hypothesis:
- On the alternative hypotheses for the win ratio, Mao (2019)
Weighted win ratio:
-
Weighted win loss approach for analyzing prioritized outcomes, Luo et al. (2017)
-
Some Meaningful Weighted Log-Rank and Weighted Win Loss Statistics, Luo and Quan (2020)
Stratified win ratio:
-
The stratified win ratio, Dong st al. (2017)
-
Adjusted win ratio with stratification: Calculation methods and interpretation, Gasparyan et al. (2020)
Dependency on follow-up time:
-
On the win-ratio statistic in clinical trials with multiple types of event, Oakes (2016)
-
Graphing the win ratio and its components over time, Finkelstein and Schoenfeld (2019)
-
Adjusting win statistics for dependent censoring, Dong et al. (2020)
-
The inverse-probability-of-censoring weighting (IPCW) adjusted win ratio statistic: an unbiased estimator in the presence of independent censoring, Dong et al. (2020)
-
The win ratio: Impact of censoring and follow‐up time and use with nonproportional hazards, Dong et al. (2020)
Win odds, handling of Ties:
-
The Win Ratio: On Interpretation and Handling of Ties, Dong (2019)
-
Win odds: An adaptation of the win ratio to include ties, Brunner (2021)
-
The use of the win odds in the design of non-inferiority clinical trials, Peng (2019)
-
The win odds: statistical inference and regression, Song (2022)
Recurrent-event win ratio
- On recurrent-event win ratio, Mao (2022)
Sample size calculation:
-
Sample size formula for general win ratio analysis, Mao (2021)
-
Sample size formula for a win ratio endpoint, Yu and Ganju (2022)
Regression:
-
A class of proportional win-fractions regression models for composite outcomes, Mao and Wang (2020)
-
Stratified Proportional Win-fractions Regression Analysis Wang and Mao (2022)
Review:
-
Statistical Models for Composite Endpoints of Death and Nonfatal Events: A Review, Mao and Kim (2021)
-
Statistical methods for composite endpoints, Hara et al. (2021)
Miscellaneous:
-
Event-specific win ratios for inference with terminal and non-terminal events, Yang (2021)
-
Win ratio approach for analyzing composite time-to-event endpoint with opposite treatment effects in its components, Liao (2022)
-
Designing a longitudinal clinical trial based on a composite endpoint: Sample size, monitoring, and adaptation, Schoenfeld (2022)
Application
Overview:
- The win ratio approach for composite endpoints: practical guidance based on previous experience, paper (2020)
Urology:
- Effect of Metabolic Syndrome on Anatomy and Function of the Lower Urinary Tract Assessed on MRI, paper (2022)
COVID-19:
-
Therapeutic versus Prophylactic Anticoagulation for Patients Admitted to Hospital with COVID-19 and Elevated D-dimer Concentration (ACTION): An Open-Label, Multicentre, Randomised, Controlled Trial, paper (2021)
-
Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): a randomised, double-blind, placebo-controlled, phase 3 trial, paper (2021)
-
Dapagliflozin in patients with COVID-19: truth or dare, paper (2021)
Oncology:
-
Breast Cancer Index Predicts Extended Endocrine Benefit to Individualize Selection of Patients with HR+ Early-stage Breast Cancer for 10 Years of Endocrine Therapy, paper (2022)
-
Investigations of methods for multiple time-to-event endpoints: A chronic myeloid leukemia data analysis, paper (2022)
-
Comparing Minimally Invasive and Open Pancreaticoduodenectomy for the Treatment of Pancreatic Cancer: a Win Ratio Analysis, paper(2022)
Cardiovascular Disease related clinical trial:
-
Influenza vaccination strategy in acute coronary syndromes: the VIP-ACS trial, paper (2022)
-
Sacubitril/valsartan versus ramipril for patients with acute myocardial infarction: win-ratio analysis of the PARADISE-MI trial, paper (2022)
-
Patiromer for the management of hyperkalemia in heart failure with reduced ejection fraction: the DIAMOND trial, paper (2022)
-
Efficacy and Safety of Empagliflozin in Hospitalized Heart Failure Patients: Main Results from The Empulse Trial, paper (2022)
-
Effects of Empagliflozin on Symptoms, Physical Limitations, and Quality of Life in Patients Hospitalized for Acute Heart Failure: Results From the EMPULSE Trial, paper (2022)
-
EMPAGLIFLOZIN IN PATIENTS HOSPITALIZED FOR DE NOVO VERSUS DECOMPENSATED CHRONIC HEART FAILURE: INSIGHTS FROM THE EMPULSE TRIAL, paper(2022)
-
Latent Pulmonary Vascular Disease May Alter the Response to Therapeutic Atrial Shunt Device in Heart Failure, link
-
Unloading left heart with a shunt device shows no benefits in heart failure with preserved ejection fraction but supports the importance of phenotyping patients in clinical trials, paper(2022)
-
Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): a randomised, multicentre, blinded, sham-controlled trial, paper(2022)
-
The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial, paper(2022)
-
A Study of Tirzepatide (LY3298176) in Participants With Heart Failure With Preserved Ejection Fraction and Obesity (SUMMIT) (SUMMIT), Link, undergoing trial in Eli Lilly and Company, win ratio as primary outcome analysis.
-
Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): a randomised, multicentre, blinded, sham-controlled trial, paper (2022)
-
Outpatient diuretic intensification as endpoint in heart failure with preserved ejection fraction trials: an analysis from TOPCAT, paper (2021)
-
Sodium–glucose co-transporter 2 inhibition in patients hospitalized for acute decompensated heart failure: rationale for and design of the EMPULSE trial, paper (2021), sponsored by Boehringer Ingelheim.
-
Application of win ratio methodology in the Global SYMPLICITY Registry for patients with atrial fibrillation or obstructive sleep apnea , paper, (2021)
-
Use of the win ratio in cardiovascular trials, paper (2020) reanalyzed several CV trials using win ratio
-
Statistical Appraisal of 6 Recent Clinical Trials in Cardiology: JACC State-of-the-Art Review, paper (2019), described how the ATTR-ACT trial used win ratio.
-
A win ratio approach to the re-analysis of Multiple Risk Factor Intervention Trial, paper (2019), MRFIT trial.
-
Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy, paper (2018), ATTR-ACT trial, funded by Pfizer.
-
Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial, paper (2017), used a Finkelstein-Schoenfeld approach
-
Hierarchical testing of composite endpoints: applying the win ratio to percutaneous coronary intervention versus coronary artery bypass grafting in the SYNTAX trial, paper (2017), reanalyzed the results using win ratio
-
The win ratio approach to analyzing composite outcomes: An application to the EVOLVE trial, paper (2016), reanalyzed the results using win ratio
-
Applying novel methods to assess clinical outcomes: insights from the TRILOGY ACS trial, paper (2014), reanalyzed the results using win ratio
Other:
-
Hierarchical endpoint analysis using win ratio in critical care: An exploration using the balanced solutions in intensive care study (BaSICS), paper(2022)
-
Using the win ratio to compare laparoscopic versus open liver resection for colorectal cancer liver metastases, paper(2022)
-
Rationale and design of the safe and timely antithrombotic removal - ticagrelor (STAR-T) trial: A prospective, multi-center, double-blind, randomized controlled trial evaluating reductions in postoperative bleeding with intraoperative removal of ticagrelor by the drugsorb™-ATR device in patients undergoing cardiothoracic surgery within 48 hours from last ticagrelor dose, paper(2022)
-
Prioritised endpoints for device-based hypertension trials: the win ratio methodology., paper (2021)
-
A win ratio approach to comparing continuous non-normal outcomes in clinical trials, paper (2016), used win ratio to reanalyze two clinical trials, CHARM and PLACIDE
-
Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery, paper (2010), used a Finkelstein-Schoenfeld approach
Summary:
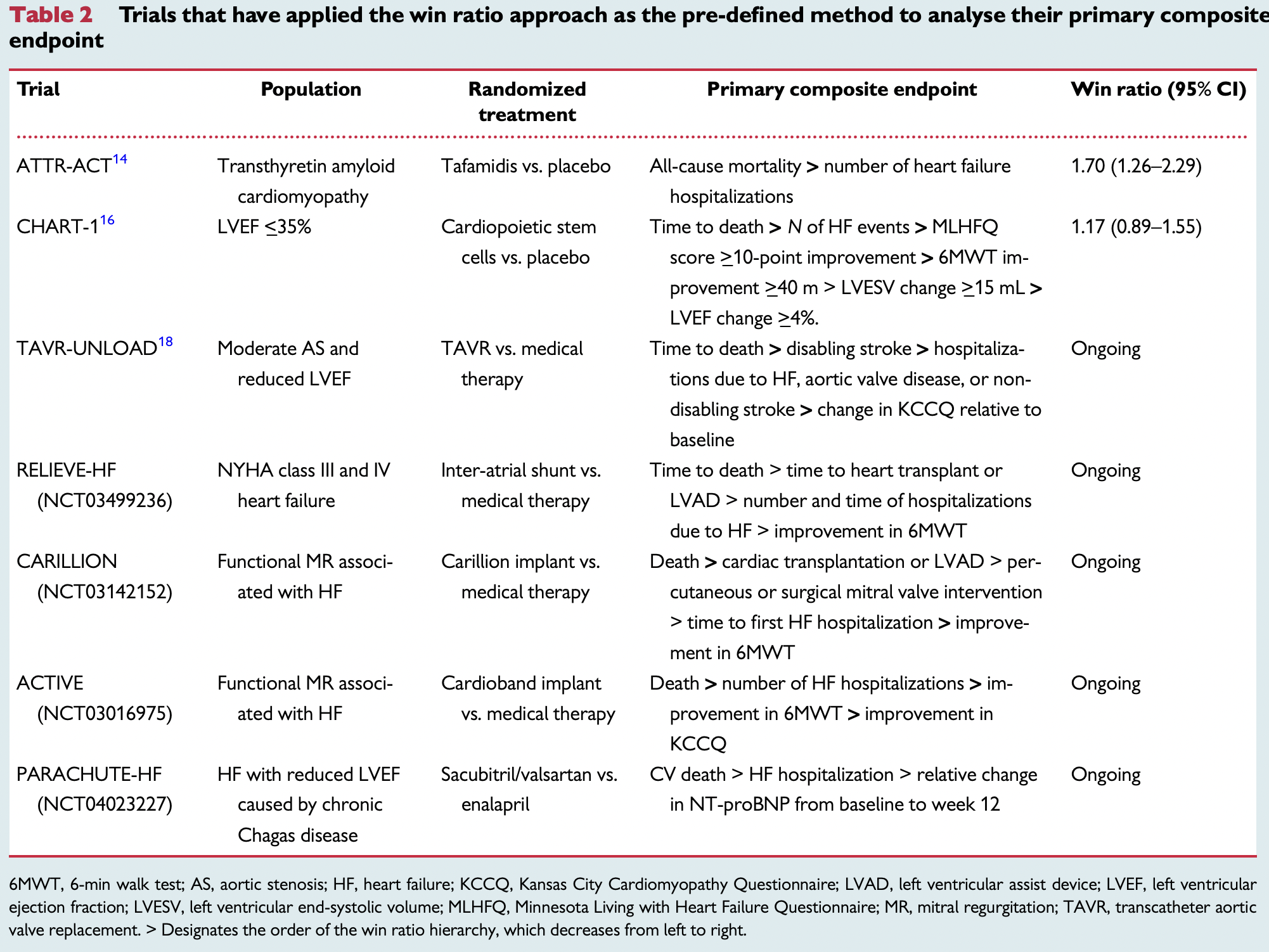
Trials that have applied the win ratio approach as the pre-defined method to analyse their primary composite endpoint. (table 2 in Redfors et al. 2020)
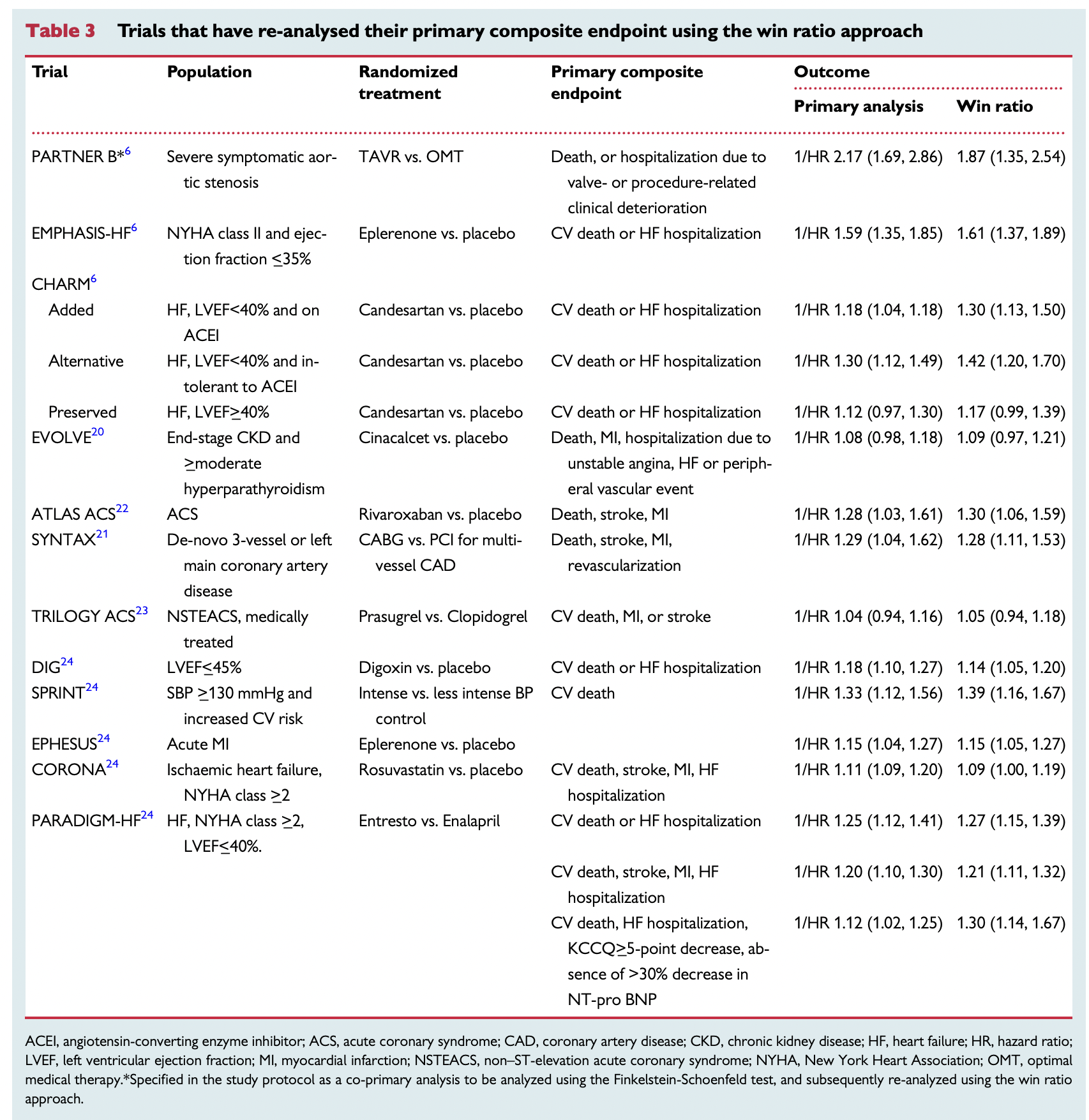
Trials that have re-analysed their primary composite endpoint using the win ratio approach. (table 3 in Redfors et al. 2020)
Software
-
Win ratio regression R package: WR
-
R package WINS
-
R package for computing confidence interval based on Bebu and Lachin (2016): WinRatio
-
R code for computing confidence interval based on Luo et al. (2015): R code
-
Supplemental material for Redfors, Björn, et al. “The win ratio approach for composite endpoints: practical guidance based on previous experience.” R code
-
Stata module to calculate the unmatched Win Ratio for prioritized outcomes, Code
-
SAS example, Code
- Posted on:
- January 15, 2021
- Length:
- 7 minute read, 1462 words
- Categories:
- Clinical Trial Survival Analysis
- See Also: